Unit chemical reactions putting it together ws 3 embarks on an illuminating journey into the fascinating realm of chemical reactions, delving into their types, balancing equations, predicting products, reaction rates, and equilibrium. This comprehensive exploration unveils the intricate workings of chemical transformations, empowering readers with a profound understanding of these fundamental processes.
Throughout this discourse, we will elucidate the mechanisms behind various reaction types, demonstrating their significance through balanced chemical equations. We will delve into the art of predicting reaction products, harnessing the power of the periodic table and chemical properties. Moreover, we will investigate the factors influencing reaction rates and delve into the concept of chemical equilibrium, exploring its implications for reaction dynamics.
Types of Unit Chemical Reactions: Unit Chemical Reactions Putting It Together Ws 3
Unit chemical reactions are classified into several types based on the changes that occur in the reactants and products. These types include:
Synthesis Reactions, Unit chemical reactions putting it together ws 3
In a synthesis reaction, two or more reactants combine to form a single product. The general form of a synthesis reaction is:
A + B → AB
Example: Hydrogen and oxygen react to form water:
2 H 2+ O 2→ 2 H 2O
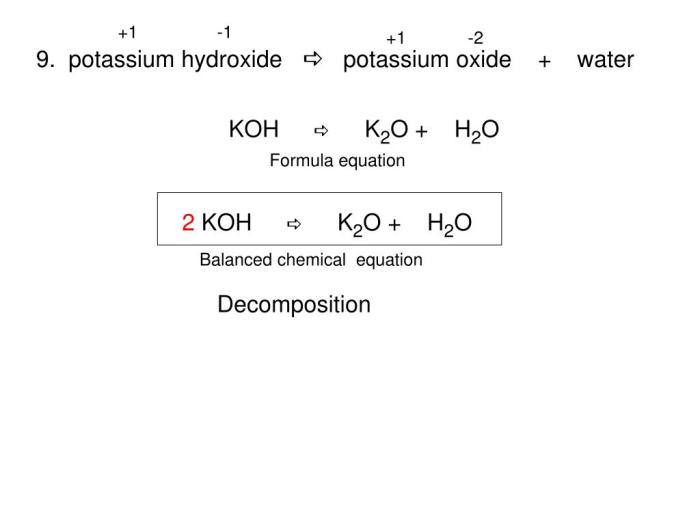
Decomposition Reactions
In a decomposition reaction, a single reactant breaks down into two or more products. The general form of a decomposition reaction is:
AB → A + B
Example: Water decomposes into hydrogen and oxygen:
2 H 2O → 2 H 2+ O 2
Single Displacement Reactions
In a single displacement reaction, one element replaces another element in a compound. The general form of a single displacement reaction is:
A + BC → AC + B
Example: Iron reacts with copper(II) sulfate to form iron(II) sulfate and copper:
Fe + CuSO 4→ FeSO 4+ Cu
Double Displacement Reactions
In a double displacement reaction, two compounds exchange ions to form two new compounds. The general form of a double displacement reaction is:
AB + CD → AD + CB
Example: Sodium chloride reacts with silver nitrate to form sodium nitrate and silver chloride:
NaCl + AgNO 3→ NaNO 3+ AgCl
Combustion Reactions
In a combustion reaction, a substance reacts with oxygen to produce heat and light. The general form of a combustion reaction is:
Fuel + O 2→ CO 2+ H 2O + heat + light
Example: Methane burns in oxygen to produce carbon dioxide and water:
CH 4+ 2 O 2→ CO 2+ 2 H 2O + heat + light
Question & Answer Hub
What are the different types of unit chemical reactions?
Unit chemical reactions encompass five primary types: synthesis, decomposition, single displacement, double displacement, and combustion.
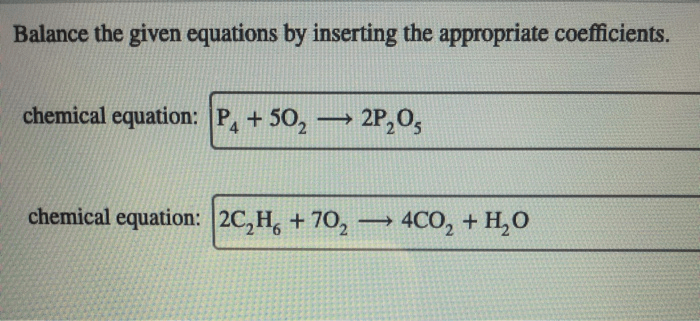
Why is balancing chemical equations important?
Balancing chemical equations ensures that the number of atoms of each element remains constant throughout a reaction, reflecting the law of conservation of mass.
How can we predict the products of chemical reactions?
Predicting reaction products involves analyzing the reactivity of elements and their positions on the periodic table, considering factors such as electronegativity and oxidation states.